Aim of the study
The main purposes of this research are to evaluate after thawing:
A) the differences of vitality (% of living cells) and other cell functions integrity of cryo-preserved cells (in particular of haematopoietic stem cells) in the presence of normal or reduced background radiations;
B) the differences of vitality and other cell functions of anucleated (with no DNA) cryo-preserved cells (in particular of erythrocytes) in the presence of normal or reduced background radiations. This model will allow to evaluate the effects on membrane lipid peroxidation and protein alteration induced by background radiations, without the most important effect that occurs at DNA level.
After thawing, vitality and cell integrity of cryo-preserved will be evaluated analysing some cellular parameters, such as: 1) cell mortality (14,17); 2) cell cycle and apoptosis (14,16-21); 3) surface antigen expression, particularly focusing on stem cell molecules and adhesion molecules regulating cell-cell interactions (22-26); 4) cytokine production (27); 5) expression of intracytoplasmic molecules such as Bcl-2 and p53 (10); 6) DNA repair capability from damages induced by chemical and physical agents (14, 16-18, 28,29).
The aim of this study is to evaluate whether, in the LNGS underground laboratory, there are advantages in conserving biological material and in particular hematopoietic stem/progenitor cells (CD34+) from cord blood or other sources. Nowadays, it is well known that stem cell transplants, necessary in some pathologies, need a high level of compatibility between donor and host. To reach this goal numerous bank of bone marrow and umbilical cord blood have been constituted. The research of a compatible donor, in the case of allogenic transplantation, may take a lot of time; on the other hand, in the case of autologous transplantation, which means with stem cells belonging to the transplanted subject himself (from umbilical cord blood or peripheral blood), stem cells have to be previously withdrawn and sometimes maintained integral for a long period. Having viability of CD34+ (hematopoietic stem/progenitor) cells important effects on the subsequent hematopoietic engraftment, we believe that it is fundamental, not only the quality of the freezing process, but also the environment in which cells are preserved. The identification of an environment that allows the maintenance of frozen biological material (in particular of stem cells of which cord blood is relatively rich) for long periods (also decades) without having large cellular alterations after thawing, it would be very important for the organization of a long lasting stem cell bank. Such environment could be that, with reduced background radiation, of Gran Sasso underground laboratory.
These kind of studies could also bring light on the state of deterioration and on the safety of numerous hospital vials or bags stored for years frozen and never used.
At the same time, to obtain theoretical data about the effects of background radiations, we will perform experiments on cryopreserved cells treated with radioactive sources that could simulate background radiations.
Materials And Methods
We will use:
A) different leukaemic lines, K562 (radiation resistant), Jurkat (radiation sensitive), HL-60 (radiation sensitive, p53 negative);
B) haemotopoietic primary cells from peripheral blood and cord blood samples.
First, the cells will be expanded and frozen at -80 °C (or 193 °K) at the lab of Centro di Citometria e Citomorfologia of Urbino. Briefly, cells will be resuspended in a freezing solutions (50% FBS, DMSO 10%, 40% RPMI 1640), stocked into 2 ml vials with
5-50x106 cells/ml and frozen at -80 °C. Then the cells will be carried to LNGS in dry ice (-78,5 °C or 194,5 °K), and subsequently maintained in dewars with liquid nitrogen (GT38, Air Liquide, Paris, France), either in the LNGS underground laboratory (Special Technique Service) in low background radiation conditions either in the LNGS outside laboratory (Chemistry Service) in normal background radiation environment. Every 2 months, both cell groups will be carried to the outside laboratory (Chemistry Service) in dry ice and rapidly thawed in water bath at 37 °C (or 310 °K).
To minimize the sources of variability, experiments will be performed in triplicates by the same operator and the quantity of radiations, present in the two laboratories, will be periodically tested.
After thawing, cells from both laboratories will be analysed to evaluate:
- cell mortality, soon after thawing and after an overnight incubation (37 °C, 5%
CO2, necessary to evaluate apoptosis), by mean of trypan blue coloration and cellular count with Neubauer;
- DNA strand break evaluation by TUNEL in situ or in situ nick translation (14, 15);
- Cell cycle kinetics after thawing by Propidium Iodide and/or Bromodeoxyuridine incorporation (14-16);
- apoptotic process and cell death, soon after thawing and after an overnight incubation (37 °C, 5%
CO2, necessary to evaluate apoptosis), by flow cytometric analysis (ethanol/propidium iodide or, if possible, annexin-V/propidium iodide (14, 17,18)], DNA analysis by elettrophoresis (ladder) and TUNEL (14, 15), and morphological analysis by TEM;
- cell cycle and the protein correlated [in particular p53 (10,14, 17,18)];
- surface antigen expression, with particular attention to stem cell molecules (CD34, CD133, CD90, CD135, CD117) and adhesion molecules in particular stem cell antigens some cytokines and the adhesion molecules (CD11a, CD11b, CD11c, CD2, CD54) (22-26);
- intracellular cytokine production (27);
- ability to repair DNA damages induced by chemical (camptothecin) and physical (ultraviolet rays) agents by mean of cell cycle analysis in flow cytometry (14, 16-18, 28,29);
- Bcl-2 expression and, if it is useful, cell membrane peroxidative state using fluorescent markers in flow cytometry (10).
The experiment that we propose to perform in underground LNGS lab is simple and well verifiable. In fact, different aliquots of the same sample of cells will be analysed by the same operator using same reagents and same instrumental analysis. For what concern the part of experiment that have to be performed in the underground LNGS lab, a very limited space will be required (the dimension of the GT38 dewar, see Fig. 1) because, in that lab, biohazard material will not be manipulated. Fig.1 Dewar GT38, Air Liquide, Paris, France
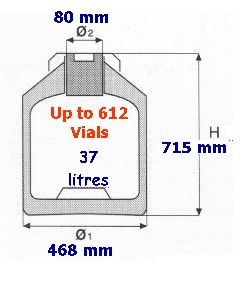
Fig.1 Dewar GT38, Air Liquide, Paris, France
Personnel and equipment required
A ) The personnel required for the evaluation of cryo-preserved cells has to be properly trained to cell culture techniques (cell culture and cell expansion, freezing and thawing) and in case to flow cytometric analysis of samples.
The following equipment and reagents are necessary:
• 1 inverted microscope for cell observation;
• 1 laminar flow hood for cell manipulation (splitting and freezing) in sterility;
• 1 CO 2 incubator for cell expansion before freezing;
• 1 thermostated bath for cell thawing (37° C);
• 1 freezer -80° C for cell gradual (initial) freezing;
• 2 dewars (GT38, Air Liquide, Paris, France) for cell cryo-maintenance; one in each laboratory (outside and underground laboratories);
• 1 bench centrifuge for washes;
• 1 mobile flow cytometer able to perform cell absolute counting (CyFLow Fig.2 , gently provided by Partec, Munster, Germany);
• 1 Neubauer's chamber for cell counting;
• material for cell cultures (sterile flasks, medium, FBS, cryo-vials, DMSO, antibiotics, glutamine, ecc.);
• various material to perform flow cytometric analysis (specific antibodies and fluorescent dies), optical fluorescent microscopy (nucleotides and kit TUNEL), and electronic microscopy (fixatives and material for inclusions).
The possibility to have a small bench cytometer for flow cytometric analysis, will allow to perform analyses directly in the LNGS outside laboratory. However, in most of the cases, cells could be transported and analyzed at the Cytometry and Cytomorphology Centre of Urbino. The cell stabilisation with TRANSFIX® will allow surface antigen analysis ( 30,31 ), while cell freezing or cell fixation with formaldehyde, ethanol or glutaraldehyde will allow DNA electrophoretic evaluation (ladder), analysis of intracytoplasmic proteins, cell cycle, DNA (TUNEL) and cell morphology (TEM), respectively.
For this reason, a permanent work at the LNGS underground laboratory, will not be necessary and thus most of the activities will be performed at the LNGS outside laboratory or at the Cytometry and Cytomorphology Centre in Urbino. This latter is a reference centre at an international level for the flow cytometric evaluation of the cell mortality and for the analysis of the haematopoietic stem cells (see pubblications 14-30 ).
Fig.2 Mobile flow cytometer able to perform cell absolute counting (CyFLow , Partec, Germany)