
The name Pulex (the latin word for flea, stemming from its small size compared to the big experiments, like Opera or Borexino etc.) does not indicate a single experiment, but rather a series of experiments, actually five up to now, whose aim is to detect if the environmental radiation did have an influence on the evolution of living matter.
Life has evolved on Earth for 3 billion years in the presence of environmental ionizing radiation. Living organisms may keep memory of being in the presence of ultra low dose rate radiation, so that a question arises as to how life on Earth or the biochemical behavior of living organisms would differ in the absence of radiation [ Todd, 1994; Yang et al., 1994].
To address this scientific question, an ideal experimental design would consist in the twin set-up of a cell or animal culture in a laboratory where environmental radiation is reduced as low as possible, and in a reference laboratory at ‘normal’ environmental levels. Using a number of experimental assays, the experimenter would monitor the biological model for the onset of any differential behavior between the two laboratories.
To achieve a condition of reduced environmental radiation, a suitable location is the underground laboratory of the Italian National Institute for Nuclear Physics at Gran Sasso (LNGS/INFN), underneath at least 1,400 m of limestone rock. This unique setting allows a significant reduction in the muon flux and the virtual elimination of atmospheric showers. Inside the underground laboratory, the organic nature of limestone rock results in a greatly reduced environmental radiation; moreover, the laboratory walls are made by specifically chosen low-activity concrete. To minimize accumulation of 222Rn which would result in a substantial exposure to densely ionizing, and therefore highly biologically effective radiation, the underground laboratory is equipped with a ventilation system that captures air from outside the highway tunnel and pumps it inside the laboratories. Occasional and short-term controlled shut-down of such ventilation system can result in spikes of 222Rn activity.
A cell culture laboratory in a low radiation environment (LRE) was established in a single room building in the LNGS underground laboratory and the “reference” radiation environment laboratory was located either at the Istituto Superiore di Sanità near central Rome (RRE-ISS) or at the LNGS (RRE-LNGS) outside the mountain.
Unlike most physics experiments, our biophysics experiment is focused on measurements on biological, living systems. The terrific complexity of a living being, even at the cellular level, affects the accuracy of any experimental determination. To minimize the effects of the large number of variables involved, one needs to define a suitable experimental model which offers control over the variable of major interest, while attempting to diminish the role of potential confounders. In vitro cell culture systems are typically at an advantage over more complex animal models, and we have accordingly designed and conducted a series of experiments in increasingly complex in vitro models: from the simplest eukariotic cells, the yeast Saccharomyces cerevisiae [Satta et al., 1995], to mammalian rodent Chinese Hamster V79 line [Satta et al., 2002], to human TK6 lymphoblastoid cell line [Carbone et al., 2009; Carbone et al., 2010]. In all the experiments performed cell cultures were settled at the LER laboratory and in one of the external laboratories (SER-ISS or SER-LNGS).
Dosimetry
Sources of background radiation were divided in 222Rn decay, cosmic, and terrestrial γ-rays components. The radiation dose associated to the gamma component was measured directly using TLD detectors, whereas the 222Rn component was determined using a combination of direct 222Rn concentration measurements and a mathematical model to convert concentration levels to cellular radiation doses.
A 222Rn concentration equal to 5 Bq/m3 (or 0.125 pCi/l, averaged inside the underground Gran Sasso laboratory at the site of the cell incubator) corresponds to a dose-rate to the cells of 0.175 nGy/h. Interestingly, in case of absence of ventilation, the 222Rn concentration at this laboratory can raise in a few hours to about 100 Bq/m3.
The cosmic ray component was considered negligible for the underground LNGS laboratory, while it can be assumed to be about 30 nGy/h for both the outdoors laboratories, based on UNSCEAR data [UNSCEAR, 2000].
From the measurements, a reduction factor of about 10 or about 80 in the γ-ray activity, and a reduction factor of about 17 or about 90 for the total dose rate, at the LRE laboratory was estimated compared to that at the RRE-LNGS or at RRE-ISS laboratory, respectively (Table 1).
When needed, challenging dose was delivered using X-rays from the 6 MV medical linear accelerator of the Radiotherapy Unit, San Salvatore Hospital, Coppito, L'Aquila, at a dose rate of 2.0 Gy min-1. In these instances, exposure to normal environmental radiation, including cosmic radiation, was negligible.
Table 1: Dosimetry estimates
Background radiation component |
LRE |
RRE-LNGS |
RRE-ISS |
Cosmic rays(a) |
negligible |
30 |
30 |
All γ-rays(b) |
3.6 |
34 |
300 |
222Rn and daughters(c) |
0.17 |
0.17 |
1.7 |
Total dose rate |
3.77 |
65.7 |
331.7 |
(a)based on UNSCEAR 2000 Report, Sources, Annex E [4]; (b)TLD measurements; (c)based on the application of the model by Jostes et. al. [1991]
Results
Three radiobiological studies have been already performed. The first one [Satta et al., 1995], carried out on the yeast strain S. Cerevisiae, indicated that cultures grown inside the Gran Sasso Laboratory for up to 120 generations were more susceptible to the toxic effects, in terms of recombination and aberration frequencies, of the radiation-mimetic chemical agent methyl-methane-sulphonate (MMS), relative to cultures maintained in the “reference” radiation levels at the La Sapienza University [Fig.1].
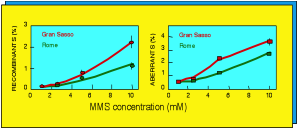
Fig. 1 Recombination and aberration frequencies in yeasts as a function on MMS concentration (adapted from Satta et al., 1995)
After such former indication, a second experiment was carried out on mammalian cells, namely Chinese hamster cells of the V79 strain, where the ”reference” radiation environment was at the Istituto Superiore di Sanità (ISS) [Satta et al., 2002]. We found that cells grown up for up to nine months in the underground laboratory of the LNGS showed an increased apoptotic activity after treatment with cycloheximide and a greater sensitivity to mutation induction by γ-rays than cells cultured at the ISS [Fig.2]. These studies supported the hypothesis of an adaptive-like response manifested in the cells cultured in the external laboratory and caused by the “reference” environmental radiation; such an adaptive-like response would be lost when cells are cultured in a significantly reduced environmental radiation.
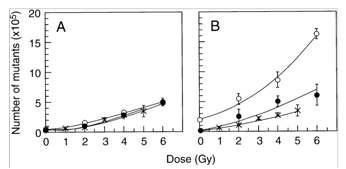
Fig. 2 Number of hprt mutants as a function of γ-ray dose. V79 Chinese hamster cells grown for 3 (panel A) and 9 (panel B) months at the ISS (closed circles) and at the LNGS (open circles). Measurements made on the starting culture (X) have been included in both panels. Each data point is the mean ±SE of 3 different determinations, performed on day 6, 8, and 10 after irradiation. (adapted from Satta et al., 2002)
To give further support to this hypothesis we investigated the biological response of human lymphoblastoid TK6 cells to acute exposures to X-rays [Carbone et al., 2009; Carbone et al., 2010]. Cultures were maintained in parallel, for 6 months, at the “reference” external laboratory of the ISS and at the “underground laboratory” of the LNGS. Measurements of DNA/chromosome damage, in terms of micronuclei induction, supported the indications provided by earlier experiments with V79 cells and yeast indicating more damage induced by a dose of 2 Gy in the cells grown in the underground laboratory (Fig.3A). Moreover, measurements showed that management of reactive oxygen species (ROS) was not the same in the two cultures. The enzymatic activity of superoxide dismutase (SOD) didn’t change so much but the catalase (CAT) and selenium-dependent glutathione peroxidase (Se-GPx) activities were significantly reduced after 6 months of cell growth in reduced radiation background environment and unable to react to the imbalance of ROS that follows acute exposure to 1 Gy of X-ray (Fig.3B).
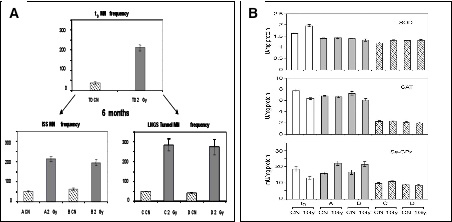
Fig 3 Panel A) Induction of micronuclei in TK6 cells maintained either at “reference”, ISS (A and B), or at low environmental radiation, LNGS (C and D). Bars represent mean of two experiments with standard error. Panel B) Antioxidant enzymatic specific activities in control (CN) and 1 h after irradiation (1 Gy X-rays) TK6 cells: at zero time (white bars, t0), after 6 months of growth at “reference” (ISS: gray bars, A and B) and at low environmental radiation (LNGS: dashed bars, C and D). Values are mean § SEM of three different experiments (adapted from Carbone et al., 2009)
Experiments have been also performed using high throughput comparative analyses of the cellular transcriptome (i.e. the set of all RNA molecules in a given condition). Using differential gene expression it was found that the cultures maintained under “reference” ionizing radiation environment acquire a transcriptome that deviates from the original, “fresh” cultures, more markedly than the transcriptome of cells maintained under reduced levels of ionizing radiation environment (Fig. 4). This is just a preliminary evidence and data analysis is still in progress.
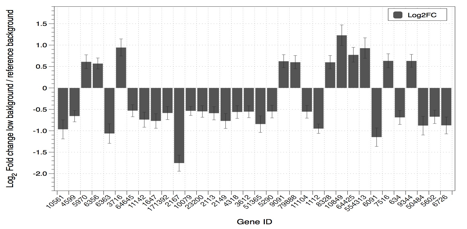
Fig 4 Example of the results obtained from gene expression analysis (quantification of fold changes ratio between cells grown for 6 months inside and outside the underground laboratory
Bibliography